Arrow in a Chemical Equation Means Which of the Following
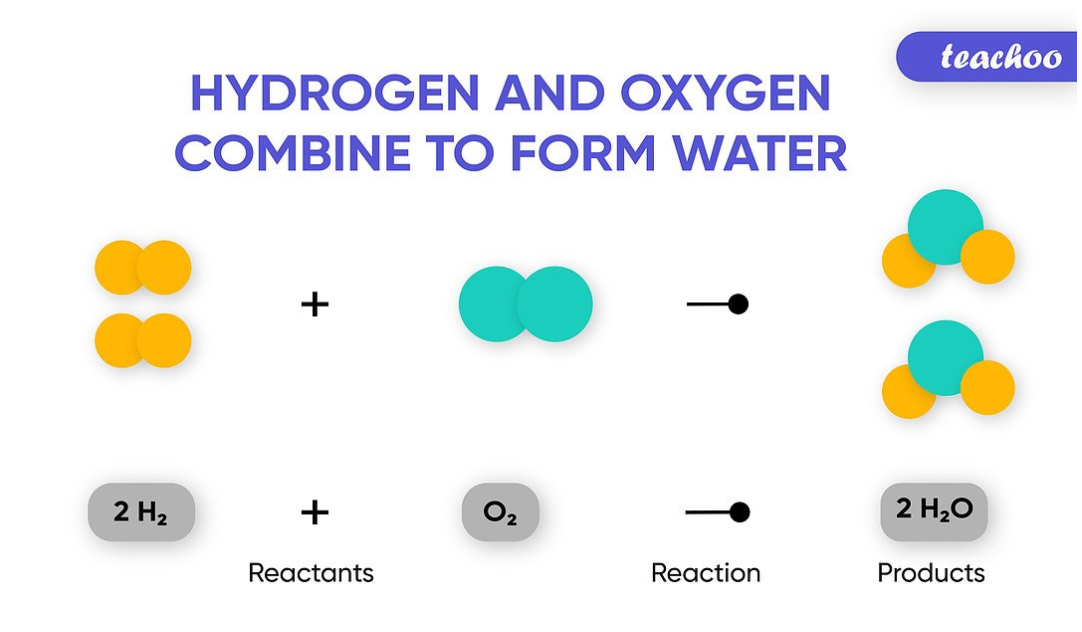
A chemical equation is interpreted as the symbolic representation of the chemical reaction where the reactants are written on the left side and the products are written on the right side. 102 Balancing Chemical Reactions Due to the Law of Conservation the number of atoms and the mass of each element must be equal on both sides of the equation.
What Does An Arrow Mean In A Chemical Equation Study Guide Inspirit
The substance which are formed after a chemical reaction are called products.

. The combination decomposition or exchange that takes place in the molecules of matter during a chemical change. That is we start with reactant and we end up with product. A word equation shows the reactant reacting to produce the product.
What is a Chemical Equation. In other words you must have the same number of each type of atom on both sides of the arrow. The arrow in the general form of the equation above shows the direction in which the chemical reaction proceeds.
The reactants and products are sunders by arrow symbols. The substance that take part in a chemical reaction are called reactants. If there are four hydrogen atoms on the left side you must.
Reactant product. A chemical reaction can be expressed in word equation and chemical equation. In order to write a word equation to represent a chemical reaction I must.
Each unique substance in the chemical reaction is sunder by a plus sign.

Question Video Recognizing What A Triangle Written Above The Arrow In A Chemical Equation Means Nagwa

Types Of Arrows Used In Chemistry Curlyarrows Chemistry Tutorials

No comments for "Arrow in a Chemical Equation Means Which of the Following"
Post a Comment